Sars Cov 2 Vaccine Vero Cell Inactivated Side Effects, Chinese Citizens Are Already Receiving A Coronavirus Vaccine The New Yorker
Muscle pain just one case of myocarditis. As you will see these points become important when we later consider what happens following a SARS-CoV-2 vaccine which is designed to induce inflammation.

Chinese Citizens Are Already Receiving A Coronavirus Vaccine The New Yorker
From 14 days after the second dose to 14 days after the third dose the levels of neutralizing antibodies of these two groups continued to rise and this trend.

Sars cov 2 vaccine vero cell inactivated side effects. Transient fever fatigue headaches andor diarrhoea. CoronaVac Suspension for Injection SARS-CoV-2 Vaccine Vero Cell Inactivated 202105064K. Pain redness swelling itching andor hardening of injection site.
The primary outcome was efficacy against laboratory-confirmed symptomatic COVID-19 14 days following a second vaccine dose among. Positive urine pregnancy test result. CoronaVac is an inactivated vaccine candidate against COVID-19 created from African green monkey kidney cells Vero cells that have been inoculated with.
Main outcomes and measures. The inactivated SARS-CoV-2 vaccine has good protective. Placebo Group Severe interstitial pneumonia 22 High viral load 22 Study showed that.
Interim analysis of 2 randomized clinical trials. SARS-CoV-2 Vaccine Vero Cell Inactivated CoronavacOn 22 February 2021 the Food and Drug Administration FDA issued authorization granting IP BIOTECH. Fever body temperature 370 dry cough fatigue.
Have a history of SARS MERS infection self-report on-site inquiry. This SARS-CorV Vero Cell Inactivated vaccine is formulated with SARS-CoV-2 strain which is inoculated in Vero cells for culturing. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes.
One concern about COVID-19 vaccines is the antibody-dependent enhancement ADE phenomenon that vaccine could make the subsequent SARS-CoV-2 infection more severe. The ensuing inflammation can lead to airway obstruction and can cause acute respiratory distress syndrome in severe cases. Subjects with history of severe allergic reactions such as acute anaphylaxis urticaria skin eczema dyspnea angioneurotic edema or abdominal pain or allergy to.
Inactivated strain of SARS-CoV-2 created from vero -cells. No systematic side effects were observed in vaccinated animals post-immunization even in at the high dose of 5000 microg. Active Systemic Anaphylaxis No allergic reaction was observed after the guinea pigs was injected.
COVID-19 immunopathology studies are still. Side Effect Common 10. Conclusions and Relevance In this prespecified interim analysis of a randomized clinical trial treatment of adults with either of 2 inactivated SARS-CoV-2.
Vaccine was administered alone to otherwise healthy control animals. The 50 microg dosage of vaccine elicited.
Sinopharm Vaccine Side Effects Price Efficacy Of China S Sinopharm Covid Vaccine India News Times Of India
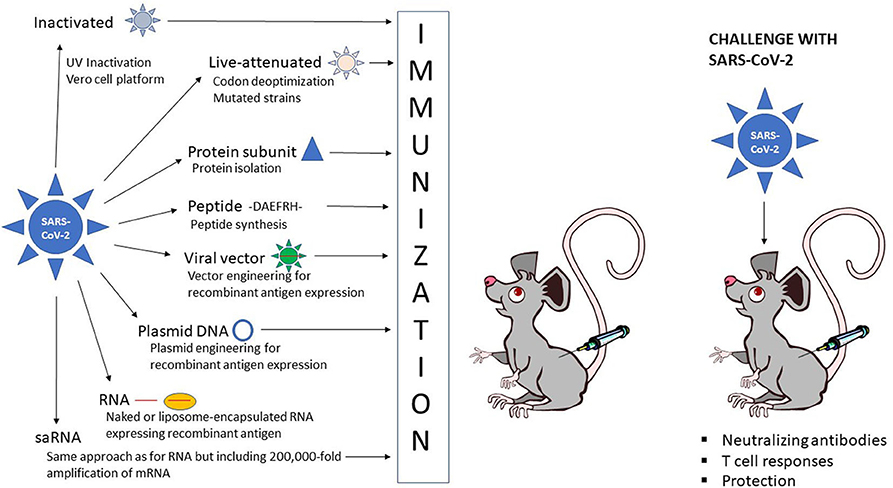
Frontiers The Current Status Of Covid 19 Vaccines Genome Editing

Sinopharm Vero Cell Inactivated Covid 19 Vaccine

Sars Cov 2 Vaccine Research And Development Conventional Vaccines And Biomimetic Nanotechnology Strategies Sciencedirect
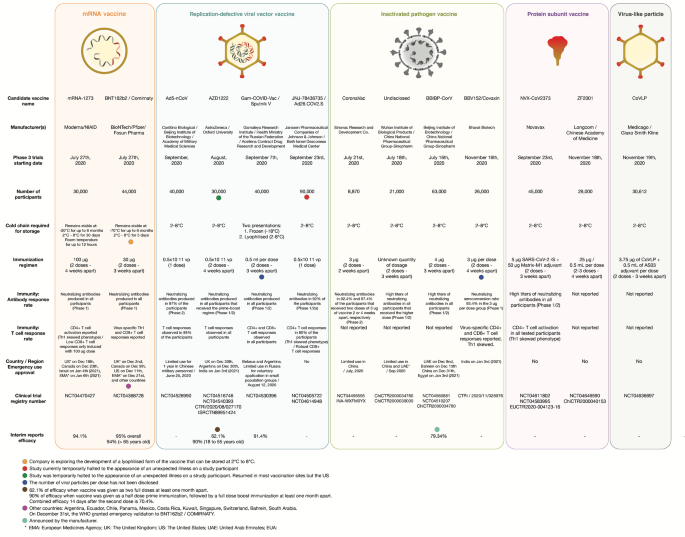
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Eu Regulator Begins Real Time Review Of First Chinese Covid 19 Vaccine Reuters
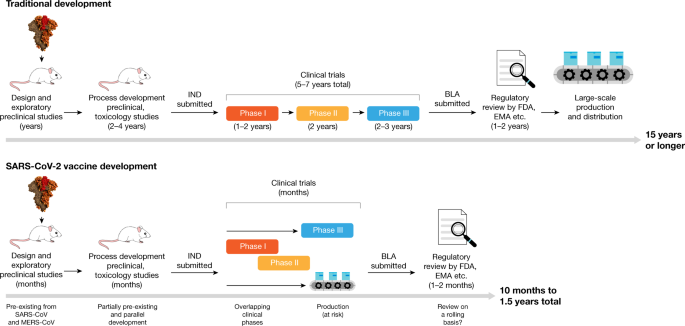
Sars Cov 2 Vaccines In Development Nature

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Development Of An Inactivated Vaccine Candidate For Sars Cov 2

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace
Covid Bolsonaro Hails Suspension Of Chinese Vaccine Trial Bbc News

Development Of An Inactivated Vaccine Candidate Bbibp Corv With Potent Protection Against Sars Cov 2 Sciencedirect

Vaccine Diplomacy Sees Egypt Roll Out Chinese Coronavirus Jab Global Health The Guardian
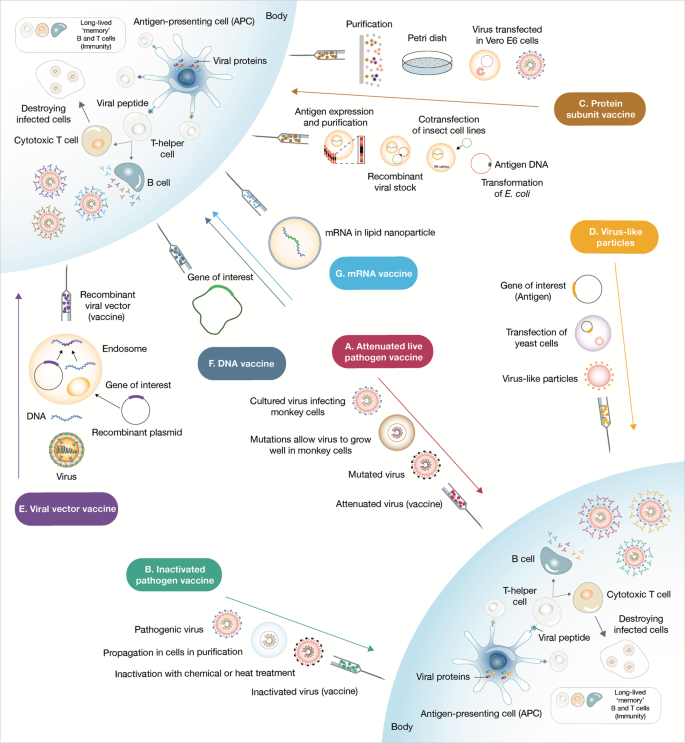
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Integrated Control Of Covid 19 In Resource Poor Countries International Journal Of Infectious Diseases
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
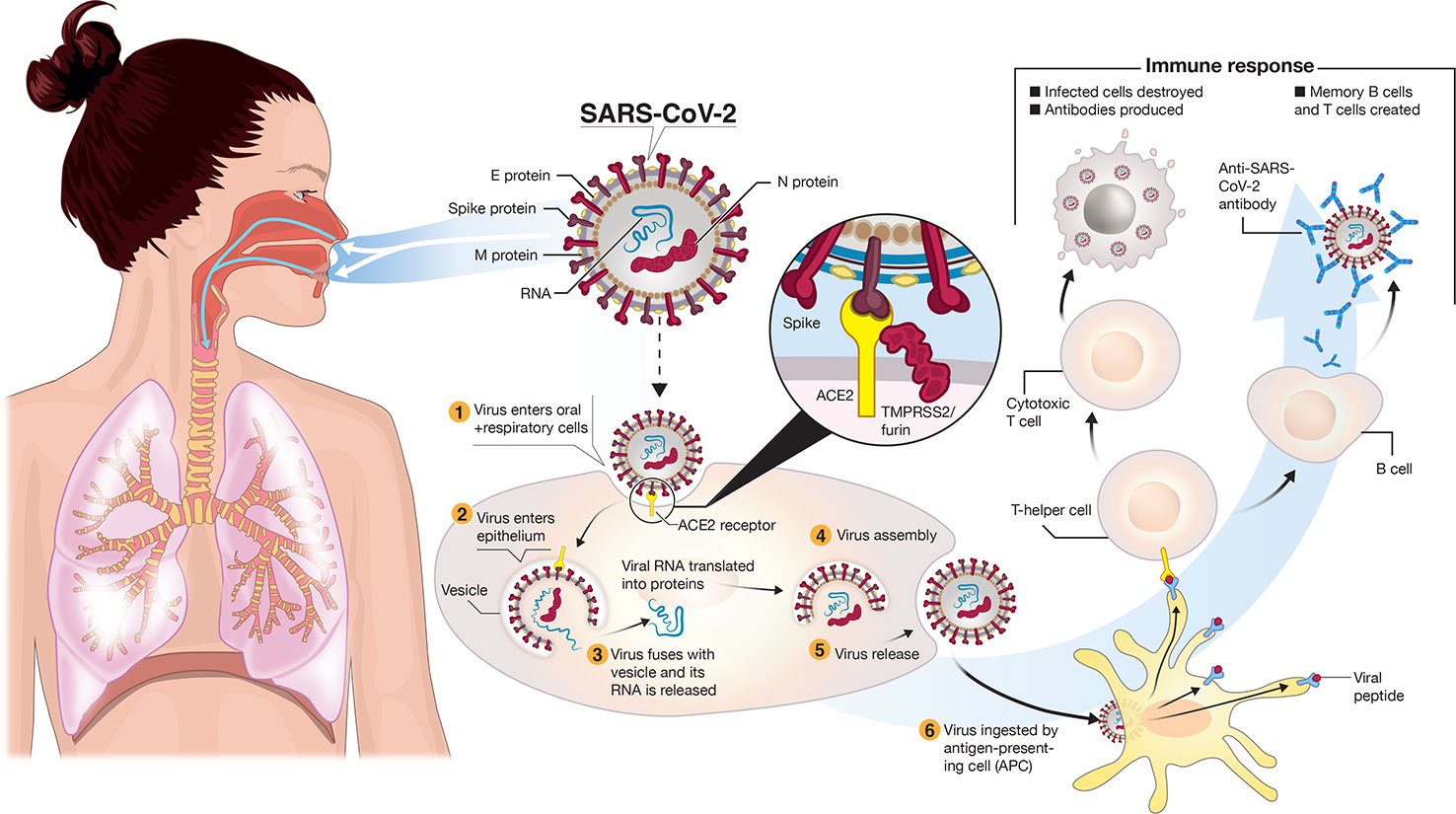
Frontiers A Snapshot Of The Global Race For Vaccines Targeting Sars Cov 2 And The Covid 19 Pandemic Pharmacology

Who Approval Of Chinese Vaccine Will Largely Accelerate Covax Supply Analysts Global Times